Bacterial vs Fungal Spores: Understanding the Titans of Contamination Control
In the high-stakes world of cleanrooms, laboratories, and GMP facilities, two microscopic challengers reign supreme: bacterial spores and fungal spores. While both can strike fear into the hearts of facility managers, understanding their differences is crucial for maintaining pristine environments and achieving regulatory compliance.
The Power Players: Not All Spores Are Created Equal
Think of bacterial spores, otherwise known as endospores, as the heavyweight champions of the microbial world. These dormant bacterial forms, like those found in C. difficile, are nature's ultimate survivors. Each endospore’s purpose is to protect the dormant bacterial spore until it encounters a suitable environment to thrive. With DNA surrounded by protective layers of macromolecules and proteins, they can withstand extreme heat, radiation, and chemical assault—making them the gold standard for validating sterilization processes. When a disinfectant can eliminate bacterial spores, it is a reasonable assumption that it can handle virtually any other contamination challenge.
Fungal spores, while still formidable, are the middleweights of the contamination world. Functioning as reproductive structures, they're primarily known for their ability to spread easily through air currents and settle on surfaces, making them a pervasive but less resistant contamination threat. However, in GMP or cleanroom environments, they can really wreak havoc when found in routine testing or remain pervasive in contamination challenges.
Why This Matters: The Spaulding Classification Perspective
The Spaulding Classification, developed over 50 years ago by Dr. E.H. Spaulding as a microbiological disinfectant hierarchy,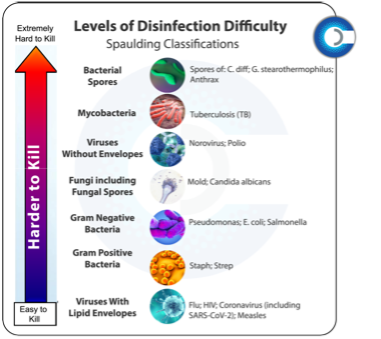 has served as an international benchmark for disinfection and sterilization requirements over the last 30 years. Notice that this model places bacterial spores at the very top of its hierarchy and CURIS’ systems are classified as this type of sporicide.
has served as an international benchmark for disinfection and sterilization requirements over the last 30 years. Notice that this model places bacterial spores at the very top of its hierarchy and CURIS’ systems are classified as this type of sporicide.
Here's why the Spaulding Classification matters:
- If a disinfectant can eliminate bacterial spores like Geobacillus stearothermophilus, it is inferred it can effectively neutralize:
- Fungal spores (like mold/aspergillus)
- Gram-positive or gram-negative bacteria (like Pseudomonas or Staphylococcus)
- Viruses (like Adenovirus), which may be moderately challenging within the body, can be easily eliminated from surfaces using proper disinfection methods like CURIS System where GLP studies prove 10-log reductions on porous and non-porous surfaces
The Daily Battle: Spore Detection and Control
Fungal Spores: The Frequent Flyers
Particle counters are commonly used in cleanrooms to detect and quantify airborne contaminants, including spores. By continuously monitoring particulate levels, facilities can promptly detect and address potential fungal contamination and ensure a controlled environment. If necessary, facilities may use PCR, the gold standard method for microbial detection, or newer bio-nano-sensor technology for identifying the presence of airborne fungal spores, which can be…
- Easily detected through particle counters
- Controlled through HEPA filtration
- Managed with regular environmental monitoring
- Indicate potential contamination when present
Bacterial Spores: The Ultimate Challenge
Due to their resilience, bacterial spores are critical for several reasons:
- Used to validate sterilization processes
- Serve as biological indicators for decontamination efficacy
- Essential for regulatory compliance
- Present a serious threat if found in critical environments
 The CURIS Advantage: Mastering Both Challenges
The CURIS Advantage: Mastering Both Challenges
What sets CURIS System's technology apart is our EPA-registered sporicide's proven efficacy against bacterial spores (6-log reduction; 99.9999%). Our low-concentration hybrid hydrogen peroxide™ technology doesn't just meet the challenge—it exceeds it. When our system can eliminate bacterial spores like C. difficile, you can trust it's more than capable of handling easier to eliminate pathogens.
Taking Action: Implementing Effective Control Measures
For facility managers and contamination control specialists, this understanding translates into action:
- Choose the Right Tools: Select EPA-registered sporicidal disinfectants or sterilants proven effective against bacterial spores
-
- If Implementing a Bio-Decontamination System: Ensure the system if federally approved as a system both solution and device:
- Ensure the solution is within expiration and record batch number and expiration date.
- Ensure in-house staff and contracted service companies are tracking reports, SOPs, and solution data to remain federally compliant.
- If Implementing a Bio-Decontamination System: Ensure the system if federally approved as a system both solution and device:
- Validate Thoroughly: Use bacterial spore indicators to confirm sterilization efficacy for bio-decontamination processes like hydrogen peroxide vapor and heat sterilization
- Monitor Continuously: Implement robust environmental monitoring for fungal spores, including use of Rodak plates and PCR testing
- Stay Compliant: Stay up to date on the latest changes in GMP and federal regulatory requirements
The Bottom Line
While both bacterial and fungal spores present significant challenges in controlled environments, bacterial spores represent the ultimate test of a disinfection system's efficacy. By choosing a solution like CURIS that's proven effective against bacterial spores, you're helping to ensure the highest level of protection against all forms of contamination.
Ready to elevate your contamination control strategy? Contact Us