Automated Material Airlocks with CURIS Decontamination Chambers
Remove human error and speed up your material transfer process while improving contamination control. CURIS hybrid hydrogen peroxide™ reaches where spray and wipe misses.
Enhance your contamination control strategy with fully automated vaporous hydrogen peroxide biodecontamination chambers. Whether you're establishing a new material transfer space or upgrading existing equipment, CURIS offers integrated solutions and retrofits to support aseptic manufacturing environments. Perfect for routine disinfection, as well as fast and easy decontamination of equipment and items moving into cleaner spaces. Fast cycles that are repeatable and robust for efficient throughput are a standard feature.
Fully Customize or Retrofit a Chamber to Meet Your Needs
CURIS provides two streamlined options to integrate advanced biodecontamination into your material airlocks (MALs) and other critical areas, facilitating efficient MAL fumigation:
Option 1: Fully Automated CURIS Decontamination Chambers
 All-in-one, custom-engineered decontamination chambers pre-installed with CURIS technology. These ready-to-use closed system, self-contained chambers are ideal for material transfer spaces, ensuring rapid and validated disinfection processes. Need to integrate into a room or facility instead? Check out our Custom Facility Integration.
All-in-one, custom-engineered decontamination chambers pre-installed with CURIS technology. These ready-to-use closed system, self-contained chambers are ideal for material transfer spaces, ensuring rapid and validated disinfection processes. Need to integrate into a room or facility instead? Check out our Custom Facility Integration.
Robust Design Meets Advanced Decontamination Technology
The CURIS Decontamination Chamber is a purpose-built, airtight enclosure designed to seamlessly integrate with CURIS' patented Hybrid Hydrogen Peroxide™ (HHP™) system. Constructed with high-grade stainless steel, the chamber ensures durability and longevity, making it ideal for rigorous use in pharmaceutical manufacturing, biotechnology, and research facilities.
Key Features:
-
Airtight Construction
Ensures complete containment, providing consistent and effective decontamination cycles. -
Integrated HHP™ Technology
Delivers a combination of vapor and micro-aerosolized hydrogen peroxide for thorough penetration and sporicidal disinfection, achieving >6-log reduction. -
Automated Operation
Features programmable cycles with integrated sensors and controls, streamlining the decontamination process to reduce human error and ensure consistent, repeatable outcomes and safety. -
User-Friendly Interface
Equipped with a touchscreen Human-Machine Interface (HMI) that allows for easy operation, monitoring, and data logging. -
Safety Prioritized
Includes built-in CURIS patented door lock technology to prevent accidentalprotect personnel by containing hybrid hydrogen peroxide™ vapor within the chamber during the decontamination process. -
Versatile Applications
Suitable for quickly decontaminating a wide range of equipment, materials, and instruments to support contamination control strategies. - Rapid CyclesDesigned with an onboard extraction system for fast load throughput, coupled with low operational PPM during decon, making this system three times faster than competing systems
Option 2: Integrated with Leading Manufacturers
CURIS systems can also be custom-engineered to integrate into leading decon chambers or sterilizers from Buxton, Getinge, PBSC, or Spire—whether you’re adding a new unit or upgrading an existing one.
Studies Proving Validated and Repeatable Results
6-Log Kill. Faster Cycles. No Compromises.
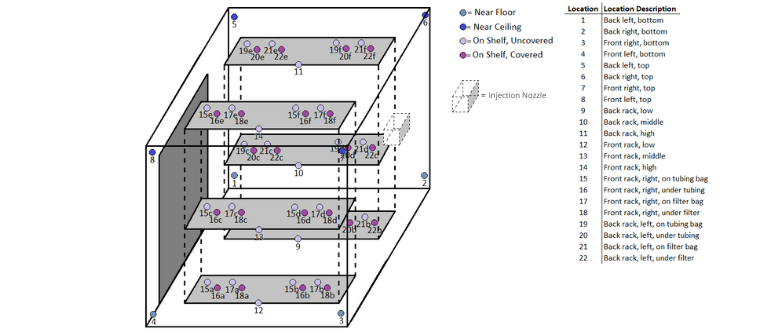 CURIS biodecontamination chambers deliver high-efficacy, rapid-cycle performance that meets regulatory standards:
CURIS biodecontamination chambers deliver high-efficacy, rapid-cycle performance that meets regulatory standards:
- Proven >6-log reduction using 7% hydrogen peroxide.
- Exceptional material compatibility with sensitive lab and production equipment.
- Validated MAL fumigation cycles ensure consistent and reliable decontamination of materials entering controlled environments.
Tailored for Your Space and Workflow
Fit Your Chamber to Your Needs, Not the Other Way Around
From chamber size and access ports to material transfer systems, CURIS works with you and your preferred chamber vendor to ensure every detail fits your operations.
Include:
- Multiple chamber sizes to accommodate various material transfer needs.
- Side or vertical loading options for ergonomic efficiency.
- Pass-through or single-door configurations to suit your facility layout.
- Optional HEPA filtration or exhaust integration for enhanced contamination control.
Move Beyond the 'Spray and Pray' Method
Are you still spraying and wiping each item when bringing them into a higher-grade space? This manual approach is time-consuming, labor-intensive, and prone to human error. Automating material transfer processes with CURIS not only saves time and labor but also enhances sterility by providing a reliable, repeatable, and validated disinfection method.
Other CURIS Custom Solutions
Need automated pass through decontamination or a system to integrate with your current BAS/BMS? Visit our Customized Integrations Page.
Trusted by biosafety and pharma leaders around the globe.
All chambers leverage CURIS’ EPA-registered 7% hybrid hydrogen peroxide™ decontamination technology.
Smart, Seamless Integration
Built-In CURIS Technology, Smarter Operation
Rapid Cycles and Aeration
Touchscreen Interface
Seamless Injection
Modular & Mobile Options
The CURIS app allows for remote operation and in-depth data management. Automated systems self-calculate treatment areas, sensors help regulate operation, and users can wirelessly sync up to 20 devices to produce valuable reports of each treatment performed. LEARN MORE