The VPHP Myth: Is High Concentration Hydrogen Peroxide Really Better than Low Concentration H2O2?

In the world of biodecontamination, one of the most persistent and misleading myths is that higher hydrogen peroxide (H2O2) concentrations automatically mean better performance. This misconception continues to be fueled by legacy biodecontamination technology built around 35% to 59% hydrogen peroxide vapor. But the science, technology, and real-world data tell a different story.
Prefer to watch instead of read? Here's a full video of this article.
Myth: High-Concentration Systems Are "The Standard"
Truth: There is no “standard” when safer, faster, and smarter methods are needed.
Over 30 years ago, with little change since, legacy systems, such as vaporized hydrogen peroxide (VHP or VPHP), were built on the assumption that high concentration was the only path to high efficacy. But today’s high-performing, low-concentration hydrogen peroxide systems by CURIS outperform legacy models1,2—delivering >6-log efficacy without the material degradation, increased human health hazards, and cycle turnover delays associated with high-concentration hydrogen peroxide. Further, federal approvals on the CURIS system and solution comply with EPA (EPA Reg. No. 93324-1), FDA, and Annex 1 standards.
Myth: Higher Concentration = Higher Efficacy; i.e., Efficacy Suffers with Low Concentration H2O2
Truth: It's not about how much, it's about how well.
Efficacy depends on delivery, distribution, and dwell time. While high concentration biodecontamination technology might sound powerful, it often comes with drawbacks: corrosive surface effects, longer cycle times, and residual vapors requiring extended aeration.3,4
 Sterilization
Sterilization
CURIS’ systems achieve a >6-log reduction of Geobacillus stearothermophilus spores—even through Penni cylinders—at a low 7% H2O2 concentration—with no added chemicals and without sacrificing efficacy.5,6 This meets and exceeds sterilization benchmarks used across the biodecontamination industry.
Repeatable Efficacy
Multiple peer-reviewed and published studies prove CURIS biodecontamination technology has superior efficacy against 10-log of adenovirus, fungi, bacterial spores, and so many more,7 including articles in the Journal of Applied Biosafety5 and mSphere.6
Myth: Low Concentration Takes Longer
Truth: CURIS fumigation systems are faster with proven efficacy.
Faster Biodecontamination Cycles
CURIS has repeatedly demonstrated full biodecontamination—quickly and thoroughly in a fraction of the time required by high-concentration systems. CURIS Rapid Vapor™ treatments, such as for fill-finish isolators, deliver complete validated biodecontamination in under one hour.6,8
 How does CURIS achieve faster efficacious cycles?
How does CURIS achieve faster efficacious cycles?
CURIS’ patented process allows vapor to permeate challenging geometries, a critical capability for pharma, bioproduction, and GMP environments. And, thanks to its low ppm operating conditions (≤135 or less) and dry application minimizing residual hydrogen peroxide, the CURIS system achieves faster aeration and a shorter overall treatment cycle (lines in blue on chart)—an efficient advantage that saves time without sacrificing efficacy.
Myth: Low-Concentration Hydrogen Peroxide Makes Surfaces Wet
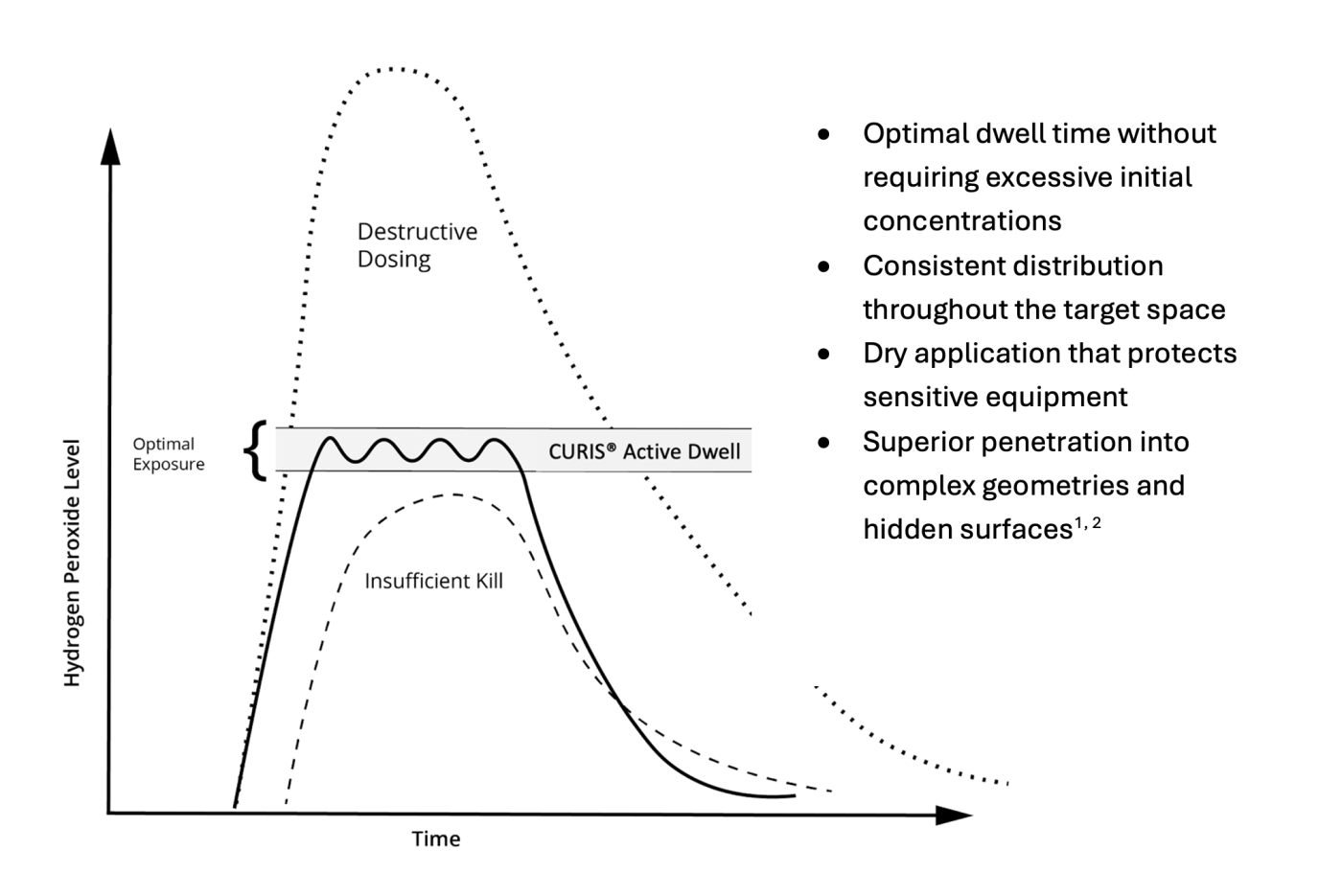 Truth: Surface residue depends on both application method and concentration.
Truth: Surface residue depends on both application method and concentration.
Low-concentration hydrogen peroxide injection systems often require wet surfaces or prolonged contact times to achieve efficacy—because they rely on a large initial dose to achieve sufficient dwell as the hydrogen peroxide naturally decomposes. However, CURIS® systems overcome that limitation through patented Pulse™ technology, delivering intermittent vaporized injections that maintain optimal concentration without over-saturating surfaces.
This dry-cycle approach ensures successful biodecontamination with minimal residue, faster aeration, and no need for post-treatment cleanup. In contrast, high-concentration systems may leave behind excess hydrogen peroxide condensation or vapor, increasing material degradation risks and extending downtime due to outgassing.
The Smarter Standard for Pharma and Bioproduction
The efficacy of CURIS’ patented technology has been validated across Class III isolators, fill-finish systems, cleanroom environments, and even through challenging soil loads. With a safer operating concentration of ≤135 ppm—compared to 400-1500 ppm+ for legacy systems—HHP™ fumigation and Rapid Vapor™ technology minimize both human hazard and material degradation while maximizing log reduction performance.5,7
Don’t Fall for the High-Concentration Hype
If your current system relies on high concentrations to achieve results, it may be time to rethink your decontamination strategy. With CURIS, you get validated performance, repeatable efficacy, safer application, and compliance-ready data—without the hazards of legacy systems.
Learn more at CURISSystem.com or contact us to schedule a demo.
Sources
1CURIS System. (2023). Material compatibility with hybrid hydrogen peroxide on critical laboratory equipment and sensors after 125 cycles of exposure. Retrieved from CURIS System documentation.
2Shih, S. Y., & Lai, C. C. (2017). Dermal injury caused by hydrogen peroxide. The Journal of Emergency Medicine, 53(6), 141–142. https://doi.org/10.1016/j.jemermed.2017.08.030 .
3Ryan, S. (2024). Effects of vapor-based decontamination systems on selected building interior materials: Vaporized hydrogen peroxide. Research Report.
4Otter, J. A., Yezli, S., Barbut, F., & Perl, T. M. (2020). An overview of automated room disinfection systems: When to use them and how to choose them. In Decontamination in hospitals and healthcare (pp. 323–369). https://doi.org/10.1016/b978-0-08-102565-9.00015-7 .
5Henneman, J. R., McQuade, E. A., Sullivan, R. R., Downard, J., Thackrah, A., & Hislop, M. (2022). Analysis of range and use of a hybrid hydrogen peroxide system for biosafety level 3 and animal biosafety level 3 agriculture laboratory decontamination. Applied Biosafety, 27(1), 7–14. https://doi.org/10.1089/apb.2021.0012.
6Derr, T. H., James, M. A., Kuny, C. V., Patel, D. R., Kandel, P. P., Field, C., Beckman, M. D., Hockett, K. L., Bates, M. A., Sutton, T. C., & Szpara, M. L. (2022). Aerosolized hydrogen peroxide decontamination of N95 respirators, with fit‑testing and viral inactivation, demonstrates feasibility for reuse during the COVID‑19 pandemic. mSphere, 7(5), e00303‑22. https://doi.org/10.1128/msphere.00303-22.
6Hultman, Carl, (Jan/Feb 2007). Physical Chemistry of Decontamination with Gaseous Hydrogen Peroxide. Pharmaceutical Engineering, 22-24.
7McAnoy, A. M., Sait, M., & Pantelidis, S. (1994). Establishment of a vaporous hydrogen peroxide bio-decontamination capability. Australian Government Department of Defense, Defense Science and Technology Organization. DSTO-TR-1994. https://apps.dtic.mil/sti/pdfs/ADA470949.pdf.
8CURIS System. (2025). Evaluation of rapid vapor™ biodecontamination in an AST fill finish isolator. CURIS Internal Report.
9Hislop, M., Grinstead, F., & Henneman, J. R. (2021). Hybrid hydrogen peroxide for viral disinfection. In R. W. Nims & M. K. Ijaz (Eds.), Disinfection of viruses. IntechOpen. https://doi.org/10.5772/intechopen.100237 .
Office of Chemical Safety and Pollution Prevention. (2020). CURoxide™ Master Label. U.S. Environmental Protection Agency. Retrieved from https://www3.epa.gov/pesticides/chem_search/ppls/093324-00001-20200716.pdf .