3 Common Misconceptions about Validation Using Spores
Misconception #1: "Mold Spores Are the Standard for Sterilization"
There's a misconception floating around that mold spores (fungal spores) are the gold standard for validating sterilization processes. Let's set the record straight: bacterial spores, not fungal spores, are the established benchmark for sterilization validation, and there's solid science behind this choice.
The confusion often stems from the high visibility of mold contamination in facilities. Yes, fungal spores are persistent and troublesome contaminants, but they're actually less resistant to sterilization processes than bacterial endospores. This is why organizations like the EPA and FDA specifically require bacterial spores for validating sterilization procedures.
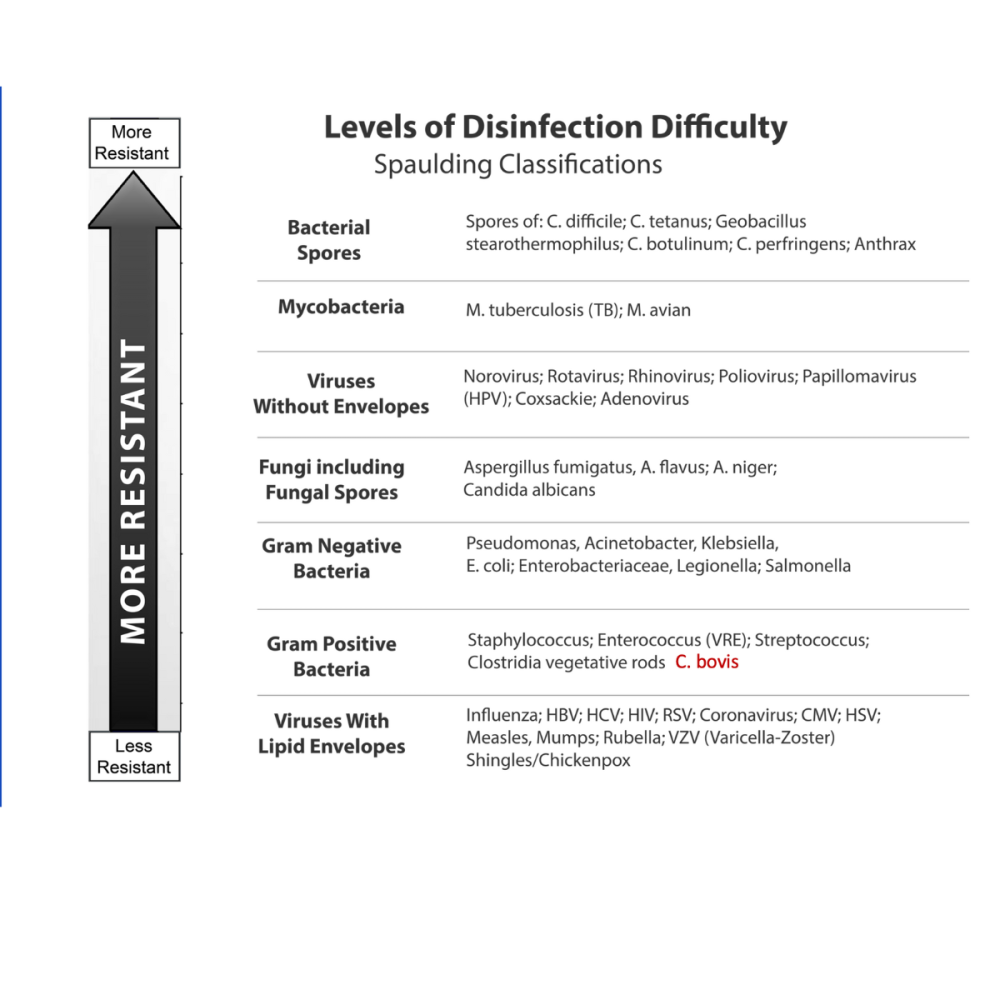
Think of it this way: if you can eliminate bacterial endospores, which are essentially microscopic fortresses with multiple protective layers around their DNA, you should reasonably be able to eliminate less resistant organisms like fungal spores. This is the principle behind the Spaulding Classification (pictured below), which has guided sterilization practices for over three decades.
Misconception #2: "A 3-Log Reduction is Sufficient for Sterilization"
One of the most dangerous misconceptions in contamination control is that achieving a 3-log reduction (99.9%) in microbial load is sufficient for sterilization. This belief can lead to serious compliance issues and potential contamination risks.
In reality, sterilization requires a much more stringent standard: a minimum 6-log reduction (99.9999%) for bacterial spores. This isn't just a regulatory requirement—it's based on the scientific understanding of microbial survival rates and the need for absolute sterility in critical environments.
To put this in perspective: if you start with 1 million bacterial spores, a 3-log reduction could still leave 1,000 viable organisms. In contrast, a 6-log reduction would leave just one organism out of that million. For truly critical applications, some facilities require even higher reductions.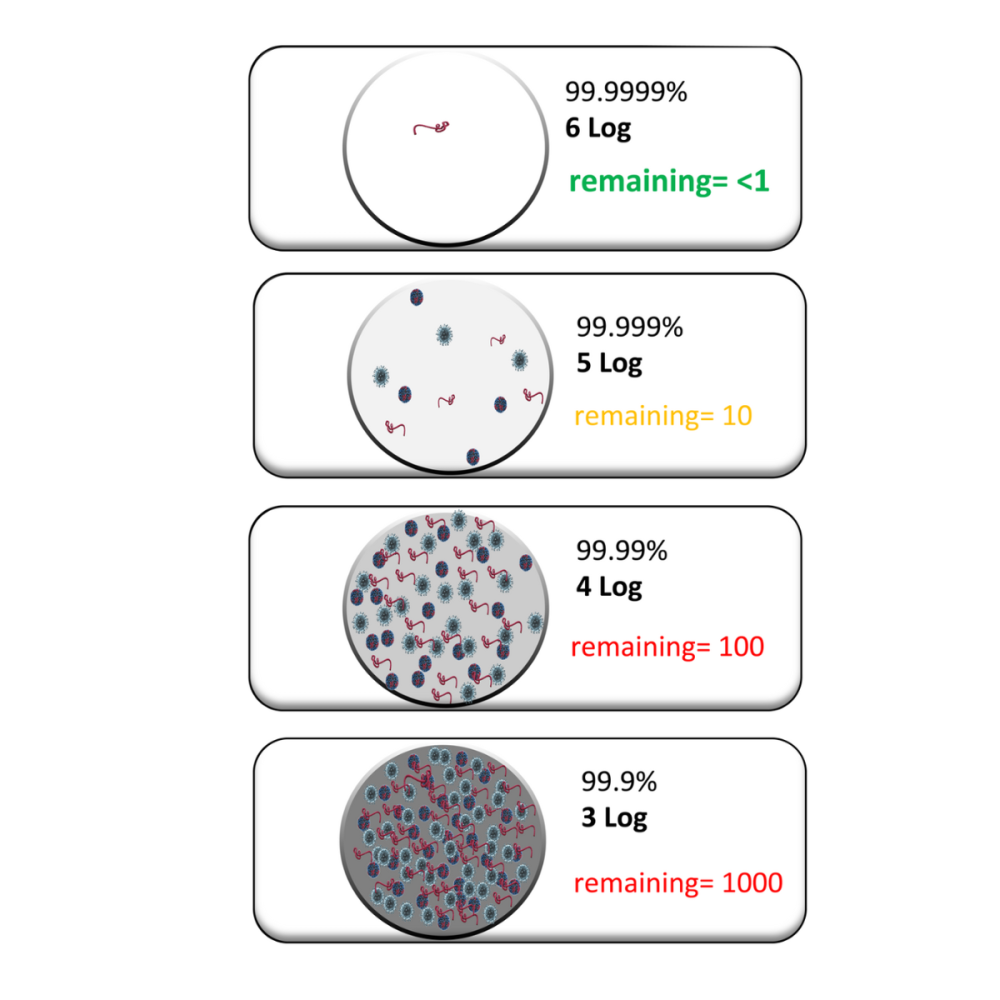
Misconception #3: "All Hydrogen Peroxide Solutions Are Equally Effective Against Spores"
A particularly persistent myth in the industry is that all hydrogen peroxide-based solutions are equally effective against bacterial spores OR that lower concentration solutions mean lower efficacy. This oversimplification can lead to material compatibility issues from high concentration systems or inadequate decontamination practices using unvetted technology and failed validation attempts.
The reality is more complex. The effectiveness of hydrogen peroxide against bacterial spores depends on multiple factors:
- The specific formulation of the solution
- The delivery method (vapor—initial injection only or Pulse™, fog, spray, etc.)
- Contact time and environmental conditions
- The presence of stabilizers, heavy metals, or other active ingredients
Some facilities learn this the hard way when theyw switch to a different hydrogen peroxide solution without proper validation, only to find their biological indicators showing incomplete sterilization. This is why it's crucial to use EPA-registered decontamination equipment with documented efficacy against bacterial spores and to validate your specific process rather than relying on general assumptions about hydrogen peroxide effectiveness.
Remember: While many hydrogen peroxide solutions may appear similar, their actual performance against bacterial spores can vary significantly. Always verify the specific claims and validation studies for your chosen solution, and ensure it meets the required log reduction standards for your application.