Applied Biosafety Journal Publishes Results of CURIS Decontamination System
CURIS System, an international manufacturer of biodecontamination solutions through its hybrid-hydrogen peroxide platform is pleased to announce a newly published study featured in the Journal of Applied Biosafety, the official journal of ABSA International (The Association for Biosafety and Biosecurity).
Applied Biosafety Journal Publishes Results of CURIS Decontamination System Demonstrating Efficacy in BSL-3 and ABSL-3Ag Facility
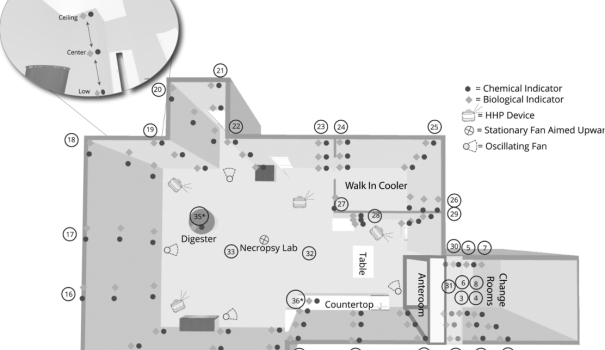
The Biosecurity Research Institute at Kansas State University tested CURIS devices in a new study proving efficacy in large necropsy suites and the interstitial spaces of laboratories. According to the study, “The Biosecurity Research Institute team sought to evaluate a novel system within their biosafety level 3 (BSL-3) and animal biosafety level 3 agriculture (ABSL-3Ag) facility.”
“Overall, this study sought to determine whether one system could conquer a range of challenges” such as reaching and decontaminating every surface in the space as well as attached rooms and decontaminating a larger open space with 21 foot ceilings which was previously perceived as ‘insurmountable.” Further, this study evaluated CURIS System’s patented technology offering the ability to operate multiple devices for automated, synchronized decontamination.
“This study is another demonstration of the six-log effectiveness of the CURIS product line. Challenging our system through peer-review and case studies demonstrates to others the value and technology CURIS products bring to their facilities. This study, and others like it, prove CURIS is an industry leader and provides a substantial, positive impact in the areas of biodecontamination, record keeping/compliance, and contamination control,” said Grinstead, CEO of CURIS System.
“Numerous research and manufacturing facilities have partnered with CURIS to produce white papers and this has helped build the impeccable reputation for the CURIS brand. It demonstrates we support and stand behind our products, fostering long-term success for the biodecontamination market,” said Hislop, Staff Biologist at CURIS System.
Authors of this study:
Biosecurity Research Institute, Kansas State University, Manhattan, Kansas, USA: John R. Henneman, Elizabeth A. McQuade, Rachael R. Sullivan, Jen Downard, and Ashley Thackrah
CURIS System Department of Science & Research: Meaghan Hislop
Published by Mary Ann Liebert, Inc.
Markets CURIS serves:
- Life Sciences/Pharma
- Healthcare
- First Responders
- Military
- Public Sector
Published online ahead of print: https://www.liebertpub.com/doi/10.1089/apb.2021.0012
About CURIS System CURIS System is a global disinfection and biodecontamination solutions provider offering a versatile suite of products made in the USA. CURIS’s EPA-registered and patented hybrid hydrogen peroxide technology has revolutionized automated, high-level disinfection across multiple markets—from portable, hand-carry, to customized, integrated disinfection technology. The CURIS app can be used for remote activation and data storage for optimal tracking, management, and reporting. Dedicated to helping facilities succeed in the battle against pathogens, CURIS is proud to provide fact-based, science-driven results with sustainable and eco-friendly technology.