Superior Alternative to ETO Sterilization

Ethylene Oxide, more commonly known as ETO, is a high-level disinfectant used for many years for sterilization of medical devices due to its ability to effectively eliminate many pathogens. However, recent investigations by the U.S. Environmental Protection Agency (EPA) and recent sustainability initiatives have caused ETO to be scrutinized due to its carcinogenic properties which can cause lymphoma and leukemia. Considering these new developments, the EPA has begun to place stringent guidelines that restrict the use of the chemical due to its health and environmental risks. While decontamination via ETO yields highly successful results, studies have proven that comparable, if not better, results can be achieved via decontamination using vapor hydrogen peroxide in low concentrations.
Hybrid Hydrogen Peroxide™— An Ideal Replacement for Ethylene Oxide Decontamination
Hydrogen peroxide is an extremely effective decontaminant that can eliminate virtually any pathogen when delivered by the proper method. However, exposure to high concentrations of hydrogen peroxide can also be very detrimental to human health. CURIS® bypasses these issues by using a 7% Hybrid Hydrogen Peroxide™ (HHP™) system. Hybrid Hydrogen Peroxide™ technology has been proven by peer-reviewed and published studies to inactivate bacterial spores at levels ≥6-log and to inactivate viruses at 10-log efficacy.
 What is CURIS HHP™ Contamination Control?
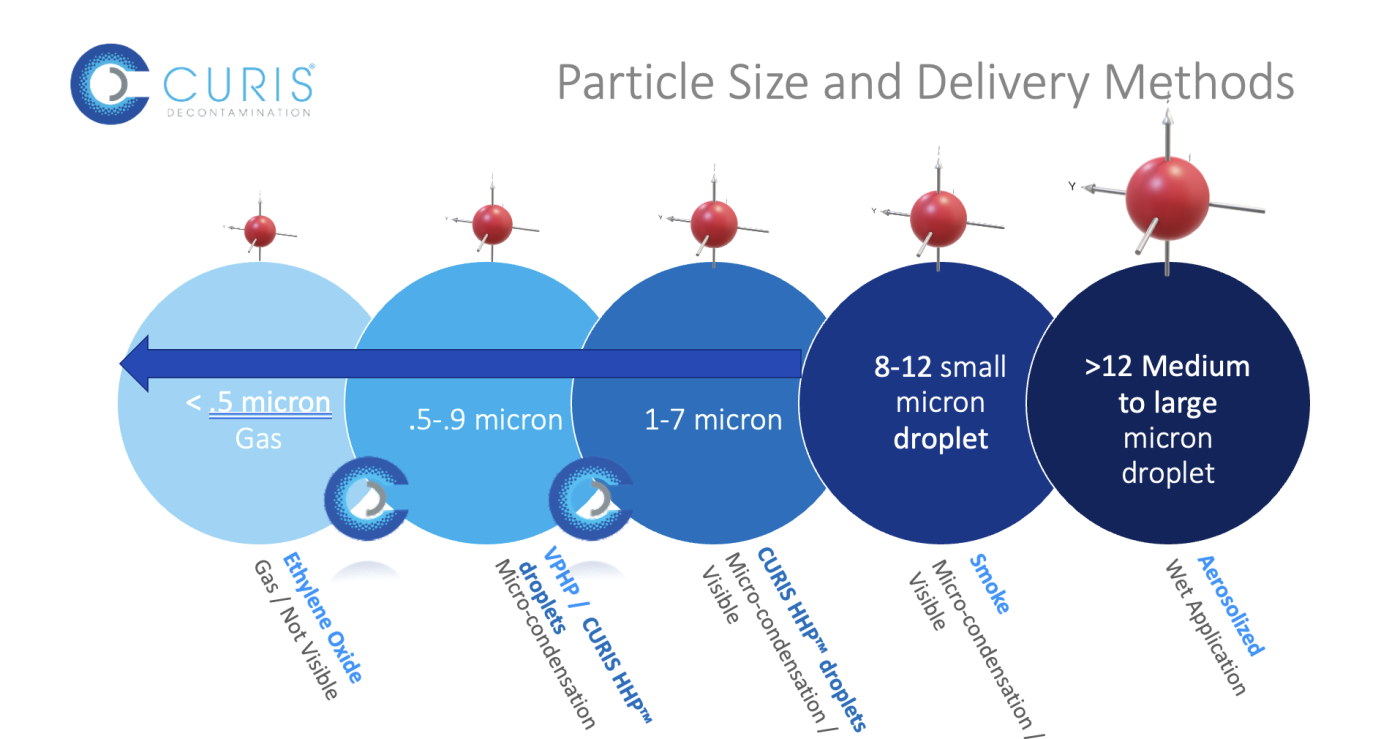
What is CURIS HHP™ Contamination Control?
CURIS HHP technology achieves high-level efficacy with a low concentration by combining vapor AND micro-aerosol H2O2, yielding extremely small particle sizes that allow the solution to remain in the air and fulfill the contact time requirement. The small particle sizes also allow for the solution to be identified as a dry applicant and prevent the hydrogen peroxide from merging into large moist droplets, thereby maintaining a dry consistent delivery.
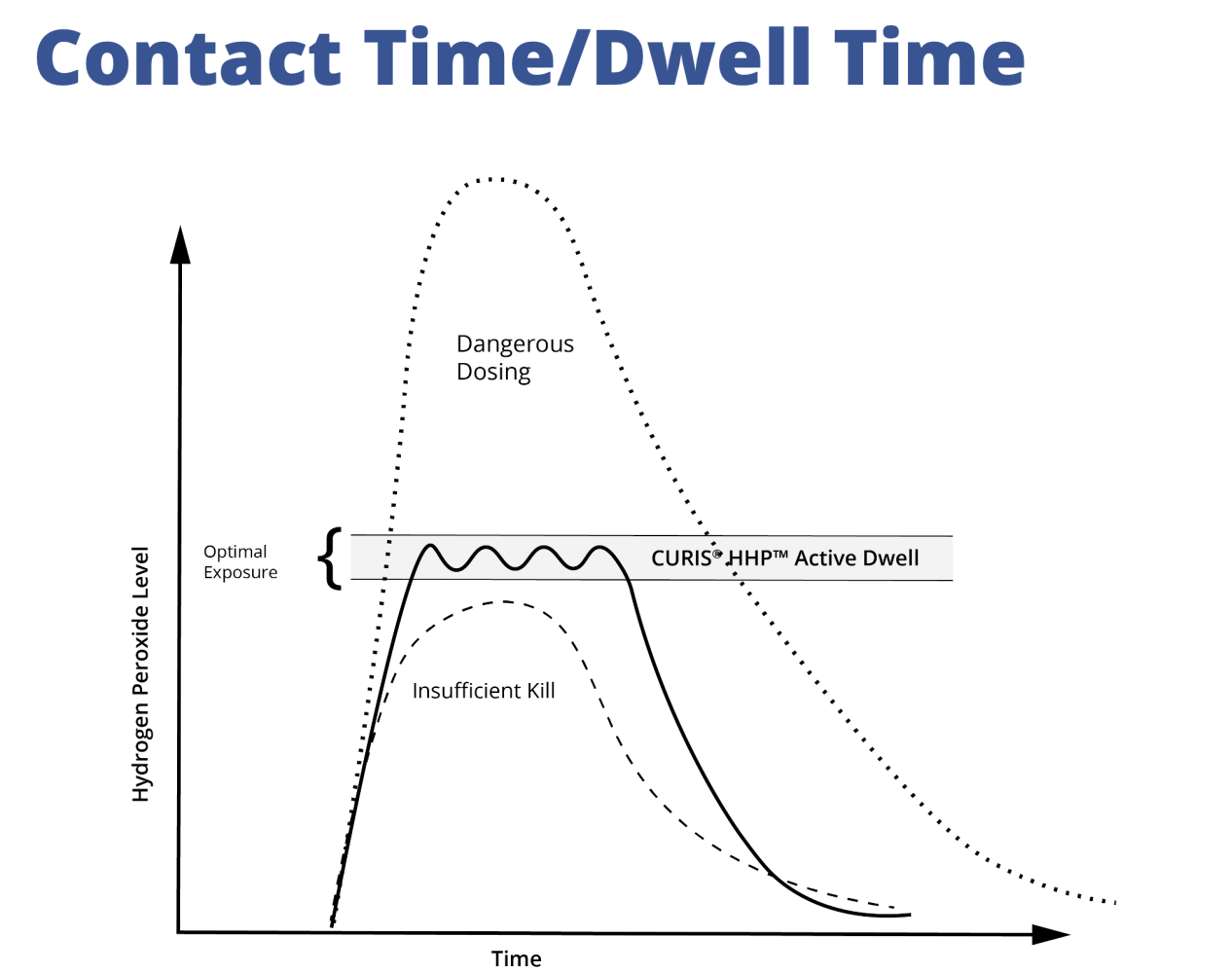
How Can CURIS® Achieve High-Level Disinfection?
CURIS’ patented Pulse™ technology replenishes any lost (decomposed) hydrogen peroxide during the cycle, which allows for efficiency and efficacy while conserving resources and maintaining an optimal active dwell period for the elimination of harmful pathogens. To further streamline the process, all CURIS devices are pre-calibrated, meaning they don’t require calibration before each use. Each device auto-calculates the proper amount of solution and time needed for each run to ensure maximum efficiency. Every system is calibrated to achieve a ≥6-log reduction (99.9999%) of bacterial spores. In fact, CURIS has been validated to achieve a 10-log (99.99999999%) reduction of adenovirus and an 8-log (99.999999%) reduction of norovirus. These high reduction rates prove CURIS has a distinctive advantage in contamination control with hydrogen peroxide.
See how CURIS can improve the contamination control process for your team—Discover CURIS’ array of decontamination devices designed to treat virtually any area in your facility.
*than 35-59% H2O2
Content provided by Gargi Paranjape, e-Pathways intern.
CURIS System is proud to support local student achievement through the e-Pathways program.